General name
Levonorgestrel
Contraindications (do not administer to the following patients)
-
- Women with a history of hypersensitivity to the ingredients of this drug
- Patients with severe liver damage
- Pregnant woman
Efficacy or effect
Emergency contraception
Precautions related to efficacy or effect
- Pregnancy cannot be completely prevented by administration of this drug.
- This drug is used urgently after sexual intercourse where contraceptive measures have failed or has not been taken, and does not systematically avoid pregnancy as with ordinary oral contraceptives.
Composition / properties
composition
Norrevo Tablets 1.5
| Active ingredient | Levonorgestrel 1.5mg in 1 tablet |
| Additive | Lactose hydrate, corn starch, povidone, light anhydrous silicic acid, magnesium stearate |
Properties of the drug
Norrevo Tablets 1.5mg
| Dosage form | White pigment lock | |
| External shape | surface |  |
| Back side |  |
|
| side | ||
| size | diameter | About 7mm |
| thickness | About 3.6mm | |
| mass | 140mg | |
| Identifying code | NL1.5 | |
Usage and dosage
A single oral dose of 1.5 mg of levonorgestrel within 72 hours after sexual intercourse.
Precautions related to usage and dosage
When administering this drug, instruct to take it as soon as possible.
Usage and dosage
A single oral dose of 1.5 mg of levonorgestrel within 72 hours after sexual intercourse.
Precautions related to usage and dosage
When administering this drug, instruct to take it as soon as possible.
Important basic notes
- When administering this drug, confirm the presence or absence of hepatic dysfunction, heart disease, renal disease and its history by interviews.
- After administration of this drug, it may not be possible to distinguish bleeding from irregular organs or bleeding in the early stages of pregnancy from menstruation. Therefore, give guidance such as returning to the hospital at an appropriate time in consideration of the menstrual cycle.
Interaction
Caution for combined use (Be careful about combined use)
| Drug name, etc. | Clinical symptoms / measures | Mechanism / risk factors |
Anticonvulsant
HIV protease inhibitor
Non-nucleoside reverse transcriptase inhibitor
Rifabutin Rifampicin |
The effect of this drug may be diminished. | These drugs are thought to induce drug-metabolizing enzymes in the liver and promote the metabolism of this drug. |
| Foods containing St. John's Wort | Be careful not to ingest foods containing St. John's wort when administering this drug, as the effect of this drug may be diminished. | This food is thought to induce drug-metabolizing enzymes in the liver and promote metabolism of this drug. |
Precautions regarding patients with specific backgrounds
Patients with complications / medical history
- Patients with heart disease or a history of it
- Symptoms may be exacerbated by retention of sodium or fluid
Patients with severe gastrointestinal disorders or malabsorption syndrome of the gastrointestinal tract
The efficacy of this drug may not be expected.
Patients with renal dysfunction
- Patients with renal disease or a history of it
- Symptoms may be exacerbated by the retention of sodium or fluid.
Patients with liver dysfunction
- Patients with severe liver damage
- Do not administer. Symptoms may be exacerbated due to decreased metabolic capacity and increased burden on the liver.
Patients with hepatic disorder (excluding patients with severe hepatic disorder)
Symptoms may be exacerbated due to decreased metabolic capacity and increased burden on the liver.
Those who have fertility
- This drug is intended to avoid pregnancy after sexual intercourse, and when planned contraception, use oral contraceptives with the highest contraceptive effect possible.
- Pregnancy may occur even after administration of this drug, so appropriate contraceptive measures should be instructed.
- When administering this drug, make sure that you are not pregnant by pelvic examination, immunological pregnancy diagnosis, etc.
Pregnant woman
Do not administer. When administered in the early or middle stages of pregnancy, virilization of the external genitalia of the female fetus or feminization of the male fetus may occur.
Milk woman
It is desirable not to breastfeed. Since the ingredients of this drug are transferred to milk, instruct to avoid breastfeeding for 24 hours after administration of this drug.
Interaction
Caution for combined use (Be careful about combined use)
| Drug name, etc. | Clinical symptoms / measures | Mechanism / risk factors |
Anticonvulsant
HIV protease inhibitor
Non-nucleoside reverse transcriptase inhibitor
Rifabutin Rifampicin |
The effect of this drug may be diminished. | These drugs are thought to induce drug-metabolizing enzymes in the liver and promote the metabolism of this drug. |
| Foods containing St. John's Wort | Be careful not to ingest foods containing St. John's wort when administering this drug, as the effect of this drug may be diminished. | This food is thought to induce drug-metabolizing enzymes in the liver and promote metabolism of this drug. |
Side effects
The following side effects may occur, so observe carefully and take appropriate measures such as discontinuing administration if any abnormalities are observed.
Other side effects
| 5% or more | 0.1-5% | Frequency unknown | |
| Psycho-nervous system | Headache (12.3%), somnolence | Dizziness, dizziness, anxiety | |
| Genital | Withdrawal bleeding (46.2%), irregular uterine bleeding (13.8%) | Many months | Menstrual delay |
| Digestive organ | nausea | Lower abdominal pain, diarrhea, abdominal pain | vomiting |
| blood | anemia | ||
| others | Malaise | Abnormal feeling, dry mouth, feeling of heat, fatigue, peripheral edema | Breast tenderness |
Precautions for application
Precautions when delivering drugs
Instruct them to take the medicine in the PTP package from the PTP sheet and take it. Accidental ingestion of the PTP sheet may cause a hard sharp corner to pierce the esophageal mucosa and further cause perforation, resulting in serious complications such as mediastinitis.
Pharmacokinetics
Blood concentration
The table below and the figures below show the pharmacokinetic parameters and plasma concentrations of a single oral dose of 2 tablets of 0.75 mg (1.5 mg as levonorgestrel) to 8 Japanese healthy adult women .
| Cmax (ng / mL) | Tmax (hr) | AUC 0-120 (ng ・ hr / mL) | T 1/2 (hr) |
| 23.87 ± 8.01 | 2.88 ± 2.03 | 435.66 ± 115.44 | 24.72 ± 3.49 |
| (Mean ± SD, n = 8) | |||
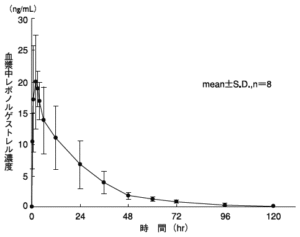
distribution
Protein binding rate
- The plasma protein binding rate was 93-95%, and the protein binding distribution was albumin 50%, SHBG (sex hormone binding globulin) and CBG (cortisol binding globulin) 48%.
Transition to milk
Twelve subjects 6-12 weeks after delivery received levonorgestrel 1.5 mg in plasma and breast milk before, 1, 2, 4, 6, 8, 24, 48, 72, 96 and 120 hours. As a result of measuring the levonorgestrel concentration, levonorgestrel rapidly transferred into milk by 2 hours after administration and peaked between 2 hours and 4 hours. After that, the levonorgestrel concentration in milk decreased to 27% of the peak 8 hours after administration and 9% of the peak 24 hours after administration. Levonorgestrel concentration transition in milk and levonorgestrel concentration transition in plasma show parallel changes over time, and the ratio of AUC 0-t of levonorgestrel concentration in milk to AUC 0-t of levonorgestrel concentration in plasma is 0.28: 1. there were
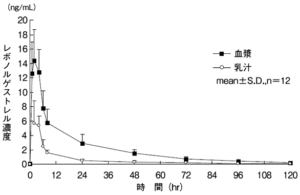
metabolism
It is metabolized in the liver to glucuronide and sulfate conjugates .
16.5 excretion
After a single oral administration of 1.5 mg of d -norgestrel (levonorgestrel) labeled with 14 C, urinary excretion was 44.8 ± 8.9% and fecal excretion was 31.6 ± 8.2% by the 7th day. Approximately 80% of the total dosed radioactivity was recovered (foreigner data).
Clinical results
17.1 Efficacy and safety testing
- 17.1.1 Domestic Phase III study
- As a result of one oral administration of 2 tablets of 0.75 mg (1.5 mg as levonorgestrel) within 72 hours after sexual intercourse, 1 of the 63 cases analyzed was pregnant and the pregnancy inhibition rate was 81.0%. ..
The frequency of adverse reactions was 72.3% (47/65), and the main adverse reactions were withdrawal bleeding 46.2% (30/65), irregular uterine bleeding 13.8% (9/65), and headache 12.3% (8/65). Examples), nausea 9.2% (6/65 cases), fatigue 7.7% (5/65 cases), somnolence 6.2% (4/65 cases), etc.
Note) Pregnancy prevention rate: A value calculated using the number of expected pregnancies obtained from the pregnancy probability for each menstrual cycle day .
Overseas Phase III study
The pregnancy rate and pregnancy inhibition rate after a single oral administration of 1.5 mg of another levonorgestrel product within 72 hours after sexual intercourse have been reported as follows.
| Date of administration | Pregnancy rate (number of pregnant cases / number of evaluated cases) |
Pregnancy prevention rate |
| 1-3 days (0-72 hours) after sexual intercourse |
1.34% (16/1198) |
84% |
The main side effects are irregular uterine bleeding 31.3% (426/1359 cases), nausea 13.9% (189/1359 cases), fatigue 13.5% (184/1359 cases), lower abdominal pain 13.5% (183/1359 cases), headache. 10.4% (142/1359 cases), floating dizziness 9.7% (132/1359 cases), breast pressure pain 8.3% (113/1359 cases), menstrual delay 4.6% (62/1359 cases).
Pharmacology
Mechanism of action
As a result of various non-clinical studies on the effects of this drug on the endometrium, decidua formation, fertilized egg implantation, cervical function, and ovulation / fertilization. It was suggested that the agent mainly exhibits a contraceptive effect by suppressing ovulation, and it is considered that the fertilization inhibitory effect and the fertilized egg implantation inhibitory effect may also be involved.
Ovulation inhibitory effect
Mature female rabbits were sterilized and mated with vas deferensed male rabbits, and a single oral dose of levonorgestrel (LNG) was administered 27 days after infertility mating. On the 28th day, the animals were mated with fertile males, slaughtered 24 hours later, and the number of ruptured follicles in the ovary and the number of eggs in the oviduct were measured. Suppression of ovulation was observed in (animals)
Actions on the endometrium
- Estradiol benzoate 2 μg / animal was repeatedly subcutaneously administered to immature female chinchilla rabbits for 8 days, and then LNG was orally administered 10 times in total over 6 days. As a result of scoring the degree of development of the uterine gland (McPhail score), the development of the uterine gland was confirmed by repeated oral administration of LNG (total dose of 60 μg / animal or more).
- Estradiol benzoate 5 μg / animal was subcutaneously administered to immature female chinchilla rabbits three times, and then LNG was administered once intrauterinely. As a result of scoring the change of the endometrium after 72 hours (endometrial finding score according to McPhail), LNG induced the secretory phase change of the endometrium in 2 out of 4 animals at 20 μg / animal .
Decidua tumor-forming action
The ovaries of female mice were removed (day 1), and the uterus was injured with nylon thread on day 6. LNG was orally administered repeatedly for 4 days from the 5th day to the 8th day, and as a result of measuring the uterine weight and histologically evaluating the dedecidua-like change on the 9th day, it dropped out at the administration of 250 μg / animal / day or more. It showed a membrane-forming effect.
Actions on cervical function
Mature female rabbits were sterilized and mated with male rabbits with vas deferens amputation. On the 26th day after infertility mating, semen collected from male rabbits was injected into the vagina, and human chorionic gonadotropin was intravenously administered to promote ovulation. LNG was continuously orally administered from the 20th day to the 27th day after infertility mating, and on the 28th day, the rabbit was slaughtered and the number of ruptured follicles in the ovary, the number of eggs in the reproductive tract and the number of fertilized eggs were measured. The fertility rate decreased at / animal / day .
Contraceptive effect
Female baboons in the maximal swelling stage of the sexual skin were placed in male baboon cages for 6 hours, and then LNG was orally administered within 6 hours. After that, as a result of observing changes in the sexual skin or menstruation, a contraceptive effect was observed by a single oral administration of 400 μg / animal of LNG to female baboons immediately after mating, but no disturbance of the sexual cycle was observed .
Physicochemical knowledge about active ingredients
General name
Levonorgestrel
Chemical name
18α-Homo-19-nor-17β-hydroxy-17α-pregn-4-en-20-yn-3-one
Molecular formula
C 21 H 28 O 2
Molecular weight
312.45
Properties
It is a white crystalline powder.
Slightly soluble in dichloromethane, sparingly soluble in ethanol, and practically insoluble in water.
Chemical structural formula
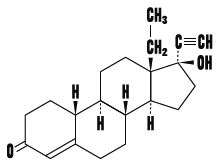
Melting point
232-239 ° C (however, the melting point range is within 4 ° C)
Handling precautions
- Put it in an outer box and store it away from direct sunlight.
- After opening the outer box, store it in the dark and use it as soon as possible.
Packaging
1 tablet [1 tablet (PTP) x 1]
Main literature
1) In-house data: Pharmacokinetic study, single dose for adult females
2) Gainer, E. et al .: Human Reproduction.2007; 22 (6): 1578-1584
3) Martindale.The Extra Pharmacopoeia 36th Ed.2009: p.2121-2122
4) Sisenwine, SFet al .: Drug Metab.Dispos.1975; 3 (3): 180-188
5) In-house data: Domestic phase III clinical trial results
6) Wilcox, AJet al .: New Engl.J.Med.1995; 333 (23): 1517-1521
7) Hertzen, H. et al .: Lancet.2002; 360 (9348): 1803-1810
8) Van der Vies, J. et al .: Arzneimittelforschung.1983; 33 (2): 231-236
9) Oettel, M. et al .: Contraception.1980; 21 (5): 537-549
Document request and contact information
Aska Pharmaceutical Co., Ltd. Medicine Counseling Room
2-5-1 Shibaura, Minato-ku, Tokyo 108-8532
TEL 0120-848-339
FAX 03-5484-8358
Precautions for insurance benefits
This drug is not covered by insurance benefits (not listed in the drug price standard).
Manufacturers, etc.
Manufacturer and distributor
Aska Pharmaceutical Co., Ltd.
2-5-1 Shibaura, Minato-ku, Tokyo
Distributor
Takeda Pharmaceutical Company Limited
4-1-1 Doshomachi, Chuo-ku, Osaka
Alliance
LABORATOIRE HRA PHARMA
