General name
Levonorgestrel ethinyl estradiol tablets
composition
It is a 21-table formulation consisting of 3 types of sugar-coated tablets: reddish brown tablets (6 tablets), white tablets (5 tablets), and pale yellowish brown tablets (10 tablets).
Active ingredient / content (in 1 tablet)
Reddish brown sugar-coated tablet levonorgestrel: 0.050 mg Japanese Pharmacopoeia
ethinyl estradiol: 0.030 mg
White sugar-coated tablet
levonorgestrel: 0.075 mg Japanese Pharmacopoeia
ethinyl estradiol: 0.040 mg
Light tan sugar-coated tablet
Levonorgestrel: 0.125 mg Japanese Pharmacopoeia
ethinyl estradiol: 0.030 mg
Additive
Reddish brown sugar-coated tablets
Corn starch, povidone, magnesium stearate, lactose hydrate, talc, titanium oxide, gum arabic powder, precipitated calcium carbonate, sucrose, sala shimitsurou, carnauba wax, hypromerose, macrogol 6000, iron sesquioxide, yellow iron sesquioxide
White sugar-coated tablets
Corn starch, povidone, magnesium stearate, lactose hydrate, talc, titanium oxide, gum arabic powder, precipitated calcium carbonate, sucrose, sala shimitsurou, carnauba wax, hypromerose, macrogol 6000
Light tan sugar-coated tablets
Corn starch, povidone, magnesium stearate, lactose hydrate, talc, titanium oxide, gum arabic powder, precipitated calcium carbonate, sucrose, sala shimitsurou, carnauba wax, hypromerose, macrogol 6000, yellow iron sesquioxide
Red sugar-coated tablets (placebo tablets)
Corn starch, povidone, magnesium stearate, lactose hydrate, talc, titanium oxide, gum arabic powder, precipitated calcium carbonate, white sugar, sardine wax, carnauba wax, red No. 102
Efficacy or effect
contraception
Precautions related to efficacy or effect
It has been reported that the failure rate in general use including forgetting to take the oral contraceptive for one year is 9%.
Usage and dosage
21 Labelfille tablets
One tablet daily is administered daily at a fixed time in a predetermined order (starting with reddish brown sugar-coated tablets) for 21 consecutive days, and the drug is withdrawn for 7 days .
The above 28 days is regarded as one administration cycle, and tablets of the next cycle are administered from the 29th day regardless of whether the bleeding has ended or continued, and the same procedure is repeated thereafter.
28 Labelfille tablets
Administer one tablet daily at a fixed time for 28 consecutive days (starting with a reddish-brown sugar-coated tablet).
The above 28 days is regarded as one administration cycle, and tablets of the next cycle are administered from the 29th day regardless of whether the bleeding has ended or continued, and the same procedure is repeated thereafter.
Precautions related to usage and dosage
- Take at a fixed time every day. (See "Important Basic Precautions")
- Start date
If you are taking an oral contraceptive for the first time, start taking it on the first day of your menstrual period. If the start date is delayed from the first day of menstruation, use other contraceptive methods for the first week of taking the drug.
Precautions for use
Careful administration
(Carefully administer to the following patients)
(Carefully administer to the following patients or women)
- Women over 40 years old [Generally, this is the age at which cardiovascular disorders such as myocardial infarction are more likely to occur, so this may be promoted. ]
- Patients with uterine fibroids [May promote the growth of uterine fibroids. ]
- Women with a history of breast cancer [Breast cancer may recur. ]
- Women with a family history of breast cancer or breast nodules [Since there are reports suggesting a causal relationship between estrogen administration and breast cancer development, it should be administered with caution, such as regular breast examinations. ]
- Smokers (see section 5 of "Contraindications")
- Obese women [It has been reported that cardiovascular disorders such as thrombosis are more likely to occur. ]
- Women with a family history of thrombosis [It has been reported that cardiovascular disorders such as thrombosis are more likely to occur. ]
- Patients with migraine without aura [It has been reported that cerebrovascular accidents (stroke, etc.) are more likely to occur. ]
- Patients with valvular heart disease (see "Contraindications" section 7.)
- Patients with mild hypertension (including a history of hypertension during pregnancy) (see "Contraindications" section 15.)
- Women with impaired glucose tolerance (diabetics and women with impaired glucose tolerance) [Since glucose tolerance may decrease, administer with sufficient control. ]
- Patients with porphyria [Symptoms may worsen. ]
- Patients with hepatic disorder (see "Contraindications" section 12.)
- Patients with heart disease, renal disease or a history of it [Symptoms may be exacerbated by retention of sodium or fluid. ]
- Epilepsy patients [Symptoms may worsen. ]
- Patients with tetany [Symptoms may worsen. ]
Important basic notes
- Thrombosis may occur regardless of the presence or absence of risk factors such as age, smoking, obesity, and family history . If any of the following symptoms occur, administration should be discontinued immediately and appropriate. Performing the treatment.
Main symptoms of thrombosis requiring urgent response Sudden pain / swelling of lower limbs, sudden shortness of breath, chest pain
, severe headache, weakness / paralysis of limbs, dysarthria, acute visual impairment, etc.
If you experience any of these symptoms, stop taking the drug immediately and explain to an emergency medical institution.
- If symptoms of suspected thrombosis occur while taking this drug, take appropriate measures such as discontinuing administration.
Symptoms of suspected thrombosis
Pain, swelling, numbness, redness, heat sensation, headache, nausea, vomiting, etc. in the lower limbs
- If there is an increased risk of thrombosis (immobility, marked increase in blood pressure, dehydration, etc.), appropriate measures such as discontinuation of administration should be taken.
- The following should be explained to the users of this drug at the start and continuation of administration.
・ Thrombosis can have a life-threatening course.
・ If symptoms of suspected thrombosis appear, or if the risk of thrombosis increases, stop taking the drug immediately and consult a doctor even if the symptoms / conditions are mild.
・ If you suspect thrombosis and visit another medical institution, notify your doctor of the use of this drug so that you can receive a medical examination with this drug in mind.
- If it is unavoidable that surgery is necessary while taking this drug, give due consideration to the prevention of thrombosis. (See section 11. of "Contraindications")
- It has been reported that age and smoking volume increase the risk of serious cardiovascular side effects. Therefore, instruct people taking this drug to quit smoking . (See section 5 of "Contraindications")
- When administering this drug, make sure that you are not pregnant by interviewing, pelvic examination, measuring basal body temperature, immunological pregnancy diagnosis, etc.
- Before administration of this drug, it is necessary to investigate the medical history of the user and to have a medical examination. This examination includes blood pressure measurement, breast / abdominal examination and clinical examination. In addition, a medical examination should be performed every 6 months during administration .
- Before and during administration of this drug, the pelvic organs, mainly the uterus and ovaries, should be examined at least once a year. Consider performing cervical cytopathology once a year.
- For breast cancer testing, instruct the recipient to perform a self-examination. Particular attention should be paid to women with a family history of breast cancer or breast nodules.
- When administering this drug, give sufficient guidance on how to take it so that you do not forget to take it. In the unlikely event that you miss a dose (excluding the 28-tablet red sugar-coated tablets), if you notice it by the next day, immediately take the missed tablet and have the tablet for that day taken as usual.
If you miss a dose for 2 consecutive days or more , stop taking the drug, wait for the next menstruation, and resume administration. If you miss a dose, you are more likely to become pregnant, so use another contraceptive method for that cycle.
- If vaginal bleeding develops while taking the drug, it usually disappears during continuous administration, but if it persists for a long period of time, it should be administered after confirming that it is not due to a malignant disease by examination such as vaginal cytopathology. ..
- If severe diarrhea and vomiting continue while taking this drug, malabsorption of this drug may occur, and in that case, the possibility of becoming pregnant increases. Therefore, the cycle should be combined with other contraceptive methods.
- If withdrawal bleeding does not occur for 2 consecutive cycles during administration, confirm that you are not pregnant prior to continuing administration.
- If you stop taking this drug and wish to become pregnant, it is desirable to use contraception until the menstrual cycle is restored.
- When switching from other oral contraceptives to this drug
(1) When switching from a 21-tablet type oral contraceptive
Take all the medicines you were taking before, and after a 7-day rest period, start taking this medicine continuously. If you are late in taking the drug, you may be pregnant.
(2) When switching from a 28-tablet type oral contraceptive
After taking all the medicines that were taken before, continue taking this medicine. If you are late in taking the drug, you may be pregnant.
Special description
- Oral contraceptives do not prevent HIV infection (AIDS) and other sexually transmitted diseases (eg, syphilis, genital herpes, gonorrhea, chlamydia infections, condyloma acuminata, trichomoniasis vaginalis, hepatitis B, etc.) . Fully explain to the recipient that the use of condoms is effective in preventing these infections .
If necessary, consider conducting a sexually transmitted disease test.
Contraindications
(Do not administer to the following patients) **, * (Do not administer to the following patients or women) 1. Women with a predisposition to hypersensitivity to the ingredients of this drug 2. Estrogen-dependent malignancies (eg, breast cancer, endometrial cancer), cervical cancer and suspected patients [may promote exacerbation or manifestation of the tumor. ] 3. Patients with abnormal genital bleeding whose diagnosis has not been confirmed [Suspected genital cancer. If the bleeding is due to genital cancer, it may promote the exacerbation or manifestation of the cancer. ] 4. Patients with thrombophlebitis, pulmonary embolism, cerebrovascular accident, coronary artery disease or a history of it [Blood coagulation ability may be enhanced and these symptoms may be exacerbated. ] 5. Smokers aged 35 and over 15 cigarettes a day [It has been reported that cardiovascular disorders such as myocardial infarction are more likely to occur. ] 6. Patients with migraine with aura (scintillating scotch, star-shaped flash, etc.) [It has been reported that patients with migraine with aura are more likely to develop cerebrovascular accidents (stroke, etc.) than patients without aura. be. ] 7. Patients with valvular heart disease with pulmonary hypertension or atrial fibrillation, patients with valvular heart disease with a history of subacute bacterial endocarditis [prone to cardiovascular disorders such as thrombosis] There is a report that it will be. ] 8. Diabetic patients with vascular lesions (diabetic nephropathy, diabetic retinopathy, etc.) [It has been reported that cardiovascular disorders such as thrombosis are more likely to occur. ] 9. Women with thrombophilia [It has been reported that cardiovascular disorders such as thrombosis are more likely to occur. ] 10. Patients with antiphospholipid antibody syndrome [It has been reported that cardiovascular disorders such as thrombosis are more likely to occur. ] 11. Patients within 4 weeks before surgery, within 2 weeks after surgery, within 4 weeks after childbirth, and in a long-term resting state [The blood coagulation ability may be enhanced and the risk of cardiovascular side effects may increase. ] 12. Patients with severe liver damage [Symptoms may worsen due to decreased metabolic capacity and increased burden on the liver. ] 13. Patients with liver tumor [Symptoms may worsen. ] 14. Patients with dyslipidemia [It has been reported that cardiovascular disorders such as thrombosis are more likely to occur. In addition, symptoms may be exacerbated because it may affect lipid metabolism. ] 15. Patients with hypertension (excluding patients with mild hypertension) [It has been reported that cardiovascular disorders such as thrombosis are more likely to occur. In addition, the symptoms may worsen. ] 16. Patients with otosclerosis [Symptoms may worsen. ] 17. Patients with a history of jaundice, persistent pruritus or herpes during pregnancy [Symptoms may recur. ] 18. Pregnant women or women who may be pregnant (see "Administration to pregnant women, lactating women, etc.") 19. Lactating women (see "Administration to pregnant women, lactating women, etc.") 20. Women who may not have completed bone growth [May cause premature closure of the epiphysis. ] |
Properties
Color / dosage form (number of tablets)
Reddish brown sugar-coated tablets (6 tablets), white sugar-coated tablets (5 tablets), light yellow-brown sugar-coated tablets (10 tablets), red sugar-coated tablets (placebo tablets) (7 tablets)
External shape
![]()
size
Diameter: 5.8mm
Thickness: 3.6mm
Mass: 90.0mg
Interaction
Caution for combined use
(Be careful when using together)
- Drug name, etc.
Corticosteroid
, prednisolone, etc.
Tricyclic antidepressant,
imipramine, etc.
Selegiline hydrochloride
Cyclosporin
omeprazole , etc.
Clinical symptoms / measures
The effects of these agents may be enhanced.
Mechanism / risk factors
This drug is thought to suppress the metabolism of these drugs.
- Drug name, etc.
Theophylline
tizanidine hydrochloride
Clinical symptoms / measures
Blood levels of these drugs may increase.
Mechanism / risk factors
This drug is thought to inhibit the metabolic enzymes (CYP1A2) of these drugs.
- Drug name, etc.
Rifampicin
, barbituric acid-based preparation,
phenobarbital, etc. Hydantoin
-based preparation, phenytoin
sodium , etc.
Clinical symptoms / measures
The effect of this drug may be diminished and the incidence of genital bleeding may increase.
Mechanism / risk factors
These drugs are thought to induce drug-metabolizing enzymes and promote the metabolism of this drug.
- Drug name, etc.
Tetracycline antibiotics
Tetracycline, etc.
Penicillin antibiotics
Ampicillin, etc.
Clinical symptoms / measures
The effect of this drug may be diminished and the incidence of genital bleeding may increase.
Mechanism / risk factors
It is considered that these drugs change the intestinal bacterial flora and suppress the reabsorption of this drug by enterohepatic circulation.
- Drug name, etc.
Terbinafine hydrochloride
Clinical symptoms / measures
It has been reported that menstrual disorders occurred when used in combination with a combination drug of progesterone and estrogen.
Mechanism / risk factors
Unknown mechanism
- Drug name, etc.
Gn-RH derivative
Buserelin acetate, etc.
Clinical symptoms / measures
It may diminish the action of these agents.
Mechanism / risk factors
Since these drugs show their efficacy by reducing the secretion of sex hormones, it is possible that the effects of these drugs may be diminished by administration of this drug, which is a sex hormone.
- Drug name, etc.
Hypoglycemic agent
Insulin preparation, sulfonylurea-based preparation, sulphonamide-based preparation, biguanide-based preparation, etc.
Clinical symptoms / measures
The action of hypoglycemic agents may be diminished. Carefully observe the blood glucose level and other conditions of the patient, and be careful to adjust the dose of hypoglycemic agent.
Mechanism / risk factors
This drug is thought to reduce glucose tolerance and diminish the action of hypoglycemic agents.
- Drug name, etc.
Lamotrigine
morphine
salicylic acid
Clinical symptoms / measures
Blood levels of these drugs may decrease.
Mechanism / risk factors
This drug is thought to promote glucuronidation of these drugs.
- Drug name, etc.
HIV protease inhibitor
Nerphinavir mesylate, ritonavir, darnavir, phosamprenavir (when combined with ritonavir),
ropinavir
etc. Non-nucleoside reverse transcriptase inhibitor nevirapine
Clinical symptoms / measures
The action of this drug may be diminished.
Mechanism / risk factors
The AUC of ethinyl estradiol is reduced.
- Drug name, etc.
HIV protease inhibitor
Atazanavir
Clinical symptoms / measures
The blood concentration of this drug may increase.
Mechanism / risk factors
It is thought to inhibit the metabolic enzyme (CYP3A4) of this drug.
- Drug name, etc.
Non-nucleoside reverse transcriptase inhibitor
Etravirine
Clinical symptoms / measures
The blood concentration of this drug may increase.
Mechanism / risk factors
Etravirine is thought to inhibit the metabolic enzyme (CYP2C9) of this drug.
- Drug name, etc.
Fluconazole
Clinical symptoms / measures
The blood concentration of this drug may increase.
Mechanism / risk factors
Fluconazole is thought to inhibit the metabolic enzyme (CYP3A4) of this drug.
- Drug name, etc.
Voriconazole
Clinical symptoms / measures
The blood concentration of this drug may increase.
Blood levels of voriconazole may increase.
Mechanism / risk factors
Voriconazole is thought to inhibit the metabolic enzyme (CYP3A4) of this drug.
This drug is thought to inhibit the voriconazole metabolizing enzyme (CYP2C19).
- Drug name, etc.
Acetaminophen
Clinical symptoms / measures
The blood concentration of this drug may increase.
Blood levels of acetaminophen may decrease.
Mechanism / risk factors
Acetaminophen is thought to inhibit the sulfate conjugate of ethinyl estradiol.
This drug is thought to promote glucuronidation of acetaminophen in the liver.
- Drug name, etc.
Foods containing St. John's Wort
Clinical symptoms / measures
Since the effect of this drug may be diminished and the incidence of genital bleeding may increase, care should be taken not to ingest foods containing St. John's wort when administering this drug.
Mechanism / risk factors
This food is thought to induce drug-metabolizing enzymes and promote metabolism of this drug.
Side effects
Overview of the occurrence of side effects, etc.
This drug has not been investigated, such as a drug use-results survey, to clarify the frequency of adverse drug reactions.
Serious side effects
Thrombosis (incidence unknown)
Thrombosis (limbs, lungs, heart, brain, retina, etc.) may occur, so observe carefully, and sudden pain / swelling of the lower limbs, sudden shortness of breath, chest pain, severe headache, weakness / paralysis of the limbs, If symptoms such as dyspnea and acute visual impairment appear, discontinue administration immediately and take appropriate measures.
Other side effects
- Hypersensitivity (incidence unknown)
Rash, urticaria
- Eye (incidence unknown)
Visual impairment due to impaired retinal blood flow
- Liver (incidence unknown)
Liver dysfunction, jaundice
- Electrolyte metabolism (incidence unknown)
Edema, weight gain
- Uterus (incidence unknown)
Vaginal bleeding (breakthrough bleeding, petechiae), lower abdominal pain, increased underbelly, candida vaginal inflammation
- Breast (incidence unknown)
Breast engorgement, breast pain
- Cardiovascular (incidence unknown)
Increased blood pressure, palpitation
- Digestive system (incidence unknown)
Nausea, vomiting, diarrhea, abdominal pain, stomatitis, loss of appetite, dry mouth, constipation, increased appetite
- Psycho-nervous system (incidence unknown)
Headache, dizziness, migraine, nervousness, drowsiness, depression
- Skin (incidence unknown)
Acne, eczema, brown spots, pigmentation Note 2)
- Other (incidence unknown)
Low back pain, stiff shoulders, malaise, decreased libido, elevated total cholesterol, elevated triglycerides, shortness of breath, epistaxis, fatigue, numbness
Note 1) Discontinue administration.
Note 2) Be careful not to expose yourself to sunlight for a long time.
If the above side effects are observed, appropriate measures such as discontinuation of administration should be taken.
Administration to pregnant women, lactating women, etc.
- Discontinue administration if pregnancy is confirmed. If you do not have withdrawal bleeding for 2 consecutive cycles, you may be pregnant, so check for pregnancy. [The safety of taking during pregnancy has not been established. ]
- Proper guidance should be given to lactating women, such as recommending other contraceptive methods. [A decrease in the quantity and quality of breast milk may occur. In addition, transfer to breast milk, jaundice and swelling of the breast have been reported in infants. ]
Impact on laboratory test results
Increases in serum proteins (corticoid-binding globulin, thyroxine-binding globulin, etc.) due to the action of the contained ethinyl estradiol may increase total cortisol, total T 3 , and total T 4 . Moreover, it is said that these free forms do not change. Be careful when determining these test values.
Precautions for application
At the time of drug delivery
Instruct them to take the medicine in the PTP package from the PTP sheet and take it. (It has been reported that accidental ingestion of the PTP sheet causes a hard sharp corner to pierce the esophageal mucosa and further causes perforation, resulting in serious complications such as mediastinitis.)
Other notes
- Foreign epidemiological studies have reported that the risk of venous thrombosis is 3.25 to 4.0 times higher in women taking oral contraceptives than in women not taking it. It has also been reported that the risk of venous thrombosis is highest during the first year of taking oral contraceptives. In addition, as a result of a large-scale post-marketing surveillance in a foreign country, not only when the oral contraceptive was first started, but also when the drug was resumed after a discontinuation of 4 weeks or more, or another oral contraceptive after a discontinuation of 4 weeks or more. It has been reported that the risk of venous thrombosis also increases when switching to, and the risk is particularly high for 3 months after the start of administration.
- As a result of epidemiological studies in foreign countries, it has been reported that taking oral contraceptives increases the possibility of developing breast cancer and cervical cancer.
- It has been reported that benign liver tumors occur in 3.4 per 100,000 people when oral contraceptives are taken in foreign countries for 2 years or more. In addition, rupture of the tumor may cause intra-abdominal hemorrhage. On the other hand, the incidence of malignant liver tumor (liver cancer) is extremely low, less than 1 in 1 million.
- Results have been reported suggesting malignant degeneration of the post-growth vaginal epithelium and endometrium of infants when estrogen is administered to pregnant animals (mice).
In addition, it has been reported that when administered to newborns (mice), malignant degeneration of the vaginal epithelium after growth was observed in the infants.
- In foreign countries, it has been reported that taking oral contraceptives caused deterioration of systemic lupus erythematosus (SLE), anaphylaxis, and hemolytic uremic syndrome (HUS).
- In foreign countries, it has been reported that contact lenses are not adjusted well due to changes in corneal thickness due to taking oral contraceptives, resulting in changes in visual acuity and visual field, and discomfort when wearing.
Pharmacokinetics
Bioequivalence test
(1) Reddish brown sugar-coated tablets
21 tablets of Labelfille and 28 tablets of Labelfille (reddish brown sugar-coated tablets) and 1 tablet of each (levonorgestrel 0.05 mg, ethinyl estradiol 0.03 mg) are orally administered once to healthy adult women by the crossover method, and levo in the serum. As a result of measuring the norgestrel concentration and the ethinyl estradiol concentration and performing statistical analysis on the obtained pharmacokinetic parameters (AUC, Cmax), the bioequivalence of the two agents was confirmed. 1)
1) Levonorgestrel
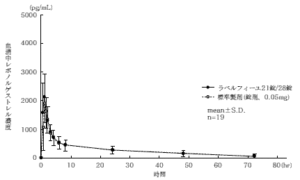
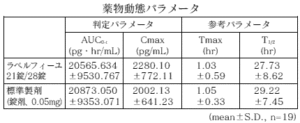
* Parameters such as serum concentration and AUC, Cmax may differ depending on the test conditions such as the selection of the subject and the number and time of body fluid collection.
2) Ethinyl estradiol
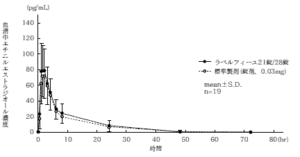
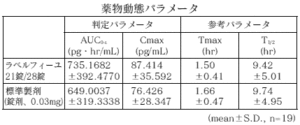
* Parameters such as serum concentration and AUC, Cmax may differ depending on the test conditions such as the selection of the subject and the number and time of body fluid collection.
(3) Light tan sugar-coated tablets
21 tablets of Labelfille and 28 tablets of Labelfille (light yellowish brown sugar-coated tablets) and 1 tablet each (levonorgestrel 0.125 mg, ethinyl estradiol 0.03 mg) were orally administered once to healthy adult women by the crossover method, and in serum. As a result of measuring the levonorgestrel concentration and ethinyl estradiol concentration and performing statistical analysis on the obtained pharmacokinetic parameters (AUC, Cmax), the bioequivalence of both agents was confirmed. 1)
1) Levonorgestrel
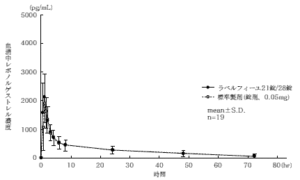
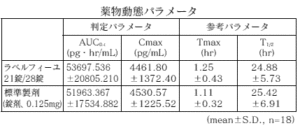
* Parameters such as serum concentration and AUC, Cmax may differ depending on the test conditions such as the selection of the subject and the number and time of body fluid collection.
2) Ethinyl estradiol
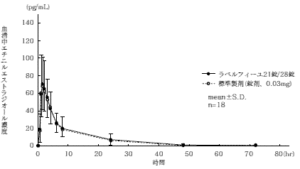
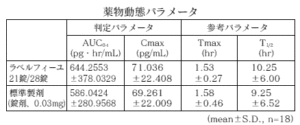
* Parameters such as serum concentration and AUC, Cmax may differ depending on the test conditions such as the selection of the subject and the number and time of body fluid collection.
Physicochemical knowledge about active ingredients
1.
common name
- Levonorgestrel
Chemical name
- (-)-13-Ethyl-17-hydroxy-18,19-dinor-17α-pregn-4-en-20-yn-3-one
Structural formula
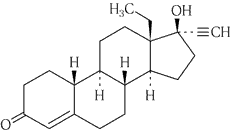
Molecular formula
C 21 H 28 O 2
Molecular weight
312.45
Properties
It is a white crystal or a crystalline powder.
It is slightly soluble in tetrahydrofuran or chloroform, sparingly soluble in methanol, ethanol (95) or acetonitrile, and practically insoluble in water.
Melting point
235 ~ 241 ℃
common name
Ethinylestradiol
Chemical name
19-Nor-17α-pregna-1,3,5 (10) -triene-20-yne-3,17-diol
Structural formula
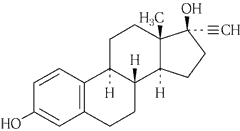
Molecular formula
C 20 H 24 O 2
Molecular weight
296.40
Properties
White to slightly yellow crystals or crystalline powder with no odor.
It is freely soluble in pyridine or tetrahydrofuran, slightly soluble in ethanol (95) or diethyl ether, and practically insoluble in water. Soluble in sodium hydroxide test solution.
Melting point
180-186 ° C or 142-146 ° C
Handling precautions
- Instruct them to keep them out of the reach of children.
- Stability test
As a result of an accelerated test (40 ± 1 ° C, relative humidity 75 ± 5%, 6 months) using the final packaged product, 21 Labelfille tablets, 28 Labelfille tablets and 28 Labelfille tablets (placebo tablets) are on the market normally. It was estimated to be stable for 3 years below. 2
Packaging
21 Labelfille: 210 tablets (PTP)
28 Labelfille tablets: 280 tablets (PTP)
Creation or revision date
** Revised June 2020 (6th edition)
* Revised in April 2016
Japanese standard product classification number
872549
Medicinal effect classification name
Oral contraceptive
Approval, etc.
Brand name
21 Labelfille tablets
Brand name code
254910BF1055
Approval / permit number
Approval number
22400AMX00481
Trademark name
Labelfille
_
NHI price standard listing date
Not listed in NHI price standard
Sales start date
June 2012
Saving method, expiration date, etc.
Saving method
Store at room temperature
Expiration date
Displayed on the outer box (3 years)
Regulation classification
Prescription drug Note)
Note) Caution — Use according to your doctor's prescription
Main documents and document request destinations
Main literature
1) Fuji Pharma Co., Ltd. in-house material (bioequivalence test)
2) Fuji Pharma Co., Ltd. in-house materials (stability test)
Document request destination
Please also request the in-house materials listed in the main documents below.
Fuji Pharma Co., Ltd. Toyama Factory Academic Information Division
1515 Mizuhashi Tsujigado, Toyama City, Toyama Prefecture 939-3515
(TEL) 076-478-0032
(FAX) 076-478-0336
Name or address of manufacturer / distributor, etc.
Manufacturer and distributor
Fuji Pharma Co., Ltd.
1515 Mizuhashi Tsujigado, Toyama City, Toyama Prefecture
